JACI:特应性皮炎治疗的潜在新方法
发布日期:2019-03-15
原标题:下一代抗-化脓性金黄色葡萄球疫苗:一种潜在的特应性皮炎治疗新方法
AD患者接种金黄色葡萄球菌疫苗可能降低高危特应性受试者的发病率,并预防或减轻其症状。这种有针对性的方法可以减少或消除广谱抗生素在抗生素耐药性增加的时代治疗金黄色葡萄球菌介导的AD炎症作用。这也可以避免对凝血酶阴性葡萄球菌的有益共生体菌株如表皮葡萄球菌和人葡萄球菌的潜在抑制,这两种菌对金黄色葡萄球菌具有抗炎和选择性抗菌活性,目前也在研究作为潜在的AD患者的治疗药物。
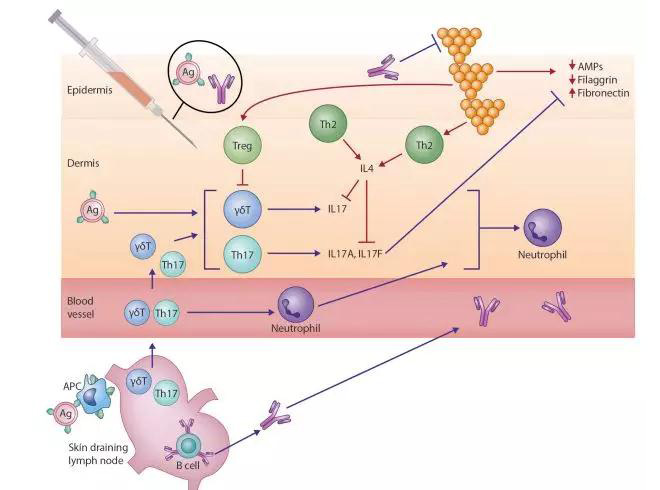
在过去的十年中,我们对AD这一复杂的异质性疾病的病因的认识有了很大的发展。“由外而内”和“由内而外”疾病过程的两分法观点已经被这样一种认识所取代,即AD的特征是受损的皮肤屏障和异常的局部和系统免疫反应的相互作用。尽管Th2偏离炎症长期以来被认为是疾病表达的中心,但TH22和TH17介导的炎症现在也在不同程度上影响不同年龄和种族的患者。金黄色葡萄球菌现在也被认为是AD患者的另一个关键致病因素,包括它在放大Th2介导反应中的关键作用。S金葡菌利用了遗传性功能缺失突变导致的聚丝蛋白表达减少,并通过TH2极化获得,以及继发于皮肤失调的抗菌肽水平降低。这些因素导致了特应性皮肤的高定植率。一旦建立,S金葡菌释放多种毒力因子,包括丝氨酸蛋白酶、外毒素和溶血素,如酚溶性调节蛋白。这些都加剧了潜在的屏障功能障碍,并使内源性促炎因子路径失调持续下去。 S金葡菌细胞壁成分,包括肽聚糖,放大TH2-驱动应答,导致增加粘附分子的表达(纤连蛋白和纤维蛋白原),减少抗菌肽的表达(人类β-防御素2和抗菌肽)和屏障蛋白(丝聚合蛋白和兜甲蛋白)。
延伸阅读
JACI Volume 143, Issue 1, January 2019, Pages 78-81
https://doi.org/10.1016/j.jaci.2018.08.038
Disease severity in patients with atopic dermatitis (AD) is directly correlated with colonization by Staphylococcus aureus.1 An increasing body of evidence now also supports a role for S aureus in the pathogenesis of AD in genetically susceptible subjects.2 Increased prevalence of S aureus preceding and coinciding with AD onset in an infant cohort suggests that early skin colonization can contribute to the development of clinical AD. However, these findings only partially explain the complex role of this organism given that another birth cohort4 did not demonstrate S aureus colonization before development of infantile AD but did show a protective effect of commensal staphylococci against later development of AD.
A vaccine against S aureus in patients with AD could potentially reduce the incidence of and prevent or attenuate symptoms in a subpopulation of high-risk atopic subjects. Such a targeted approach could reduce or eliminate the role of broad-spectrum antibiotics to treat S aureus–mediated flares of AD in an era of increasing antimicrobial resistance.5 This would also avoid the suppression of potentially beneficial commensal strains of coagulase-negative staphylococci, such as Staphylococcus epidermidis and Staphylococcus hominis, which exert anti-inflammatory and selective antimicrobial activity against S aureus and are also under investigation as potential therapeutic agents in patients with AD.
Our understanding of the cause of AD, a complex heterogenous condition, has evolved considerably in the past decade. Dichotomous views of an “outside-in” versus an “inside-out” disease process have been superseded by the recognition that AD is characterized by the interplay of both a compromised skin barrier and aberrant local and systemic immune responses. Although TH2-deviated inflammation has been long recognized as central to disease expression, TH22- and TH17-mediated inflammation are now also implicated to varying degrees across specific patient age profiles and ethnicities.7
S aureus is also now recognized as an additional key pathogenic factor in patients with AD, including its critical role in amplifying TH2-mediated responses. S aureus exploits decreased filaggrin expression resulting from inherited loss-of-function mutations and acquired through TH2 polarization and reduced antimicrobial peptide levels secondary to cutaneous dysbiosis (Fig 1).2 These factors contribute to high colonization rates in atopic skin. Once established, S aureus releases multiple virulence factors, including serine proteases, exotoxins, and lysins, such as phenol-soluble modulins. These exacerbate underlying barrier dysfunction and perpetuate endogenous dysregulated proinflammatory pathways.8S aureus cell-wall components, including peptidoglycan, amplify the TH2-driven response,8 leading to increased expression of adhesion molecules (fibronectin and fibrinogen) and reduced expression of antimicrobial peptides (human β-defensin 2 and cathelicidin antimicrobial peptide) and barrier proteins (filaggrin and loricrin).
All Author:
JulianneClowryMBabcAlan D.IrvineMD, DScabcRachel M.McLoughlinPhDd


——浙大迪迅 译
特应性皮炎(AD)患者的病情严重程度与金黄色葡萄球菌的定植直接相关。越来越多的证据也支持金黄色葡萄球菌在基因易感人群AD发病机制中的作用。在一个以婴幼儿为对象的研究组中发现,特应性皮炎发病时和/或发病前金黄色葡萄球菌的定植增加,这表明早期皮肤定植能诱发临床特应性皮炎的发生。然而,这些研究结果只能部分解释这种微生物的复杂角色,在另一个出生队列的研究中没能证实婴幼儿特应性皮炎发病前金黄色葡萄球菌的定植,相反,表现出共生葡萄球菌对以后特应性皮炎发病的保护作用。AD患者接种金黄色葡萄球菌疫苗可能降低高危特应性受试者的发病率,并预防或减轻其症状。这种有针对性的方法可以减少或消除广谱抗生素在抗生素耐药性增加的时代治疗金黄色葡萄球菌介导的AD炎症作用。这也可以避免对凝血酶阴性葡萄球菌的有益共生体菌株如表皮葡萄球菌和人葡萄球菌的潜在抑制,这两种菌对金黄色葡萄球菌具有抗炎和选择性抗菌活性,目前也在研究作为潜在的AD患者的治疗药物。
在过去的十年中,我们对AD这一复杂的异质性疾病的病因的认识有了很大的发展。“由外而内”和“由内而外”疾病过程的两分法观点已经被这样一种认识所取代,即AD的特征是受损的皮肤屏障和异常的局部和系统免疫反应的相互作用。尽管Th2偏离炎症长期以来被认为是疾病表达的中心,但TH22和TH17介导的炎症现在也在不同程度上影响不同年龄和种族的患者。金黄色葡萄球菌现在也被认为是AD患者的另一个关键致病因素,包括它在放大Th2介导反应中的关键作用。S金葡菌利用了遗传性功能缺失突变导致的聚丝蛋白表达减少,并通过TH2极化获得,以及继发于皮肤失调的抗菌肽水平降低。这些因素导致了特应性皮肤的高定植率。一旦建立,S金葡菌释放多种毒力因子,包括丝氨酸蛋白酶、外毒素和溶血素,如酚溶性调节蛋白。这些都加剧了潜在的屏障功能障碍,并使内源性促炎因子路径失调持续下去。 S金葡菌细胞壁成分,包括肽聚糖,放大TH2-驱动应答,导致增加粘附分子的表达(纤连蛋白和纤维蛋白原),减少抗菌肽的表达(人类β-防御素2和抗菌肽)和屏障蛋白(丝聚合蛋白和兜甲蛋白)。

延伸阅读
JACI Volume 143, Issue 1, January 2019, Pages 78-81
[IF:13.1]
Next-generation anti–Staphylococcus aureus vaccines: A potential new therapeutic option for atopic dermatitis?https://doi.org/10.1016/j.jaci.2018.08.038
Disease severity in patients with atopic dermatitis (AD) is directly correlated with colonization by Staphylococcus aureus.1 An increasing body of evidence now also supports a role for S aureus in the pathogenesis of AD in genetically susceptible subjects.2 Increased prevalence of S aureus preceding and coinciding with AD onset in an infant cohort suggests that early skin colonization can contribute to the development of clinical AD. However, these findings only partially explain the complex role of this organism given that another birth cohort4 did not demonstrate S aureus colonization before development of infantile AD but did show a protective effect of commensal staphylococci against later development of AD.
A vaccine against S aureus in patients with AD could potentially reduce the incidence of and prevent or attenuate symptoms in a subpopulation of high-risk atopic subjects. Such a targeted approach could reduce or eliminate the role of broad-spectrum antibiotics to treat S aureus–mediated flares of AD in an era of increasing antimicrobial resistance.5 This would also avoid the suppression of potentially beneficial commensal strains of coagulase-negative staphylococci, such as Staphylococcus epidermidis and Staphylococcus hominis, which exert anti-inflammatory and selective antimicrobial activity against S aureus and are also under investigation as potential therapeutic agents in patients with AD.
Our understanding of the cause of AD, a complex heterogenous condition, has evolved considerably in the past decade. Dichotomous views of an “outside-in” versus an “inside-out” disease process have been superseded by the recognition that AD is characterized by the interplay of both a compromised skin barrier and aberrant local and systemic immune responses. Although TH2-deviated inflammation has been long recognized as central to disease expression, TH22- and TH17-mediated inflammation are now also implicated to varying degrees across specific patient age profiles and ethnicities.7
S aureus is also now recognized as an additional key pathogenic factor in patients with AD, including its critical role in amplifying TH2-mediated responses. S aureus exploits decreased filaggrin expression resulting from inherited loss-of-function mutations and acquired through TH2 polarization and reduced antimicrobial peptide levels secondary to cutaneous dysbiosis (Fig 1).2 These factors contribute to high colonization rates in atopic skin. Once established, S aureus releases multiple virulence factors, including serine proteases, exotoxins, and lysins, such as phenol-soluble modulins. These exacerbate underlying barrier dysfunction and perpetuate endogenous dysregulated proinflammatory pathways.8S aureus cell-wall components, including peptidoglycan, amplify the TH2-driven response,8 leading to increased expression of adhesion molecules (fibronectin and fibrinogen) and reduced expression of antimicrobial peptides (human β-defensin 2 and cathelicidin antimicrobial peptide) and barrier proteins (filaggrin and loricrin).
All Author:
JulianneClowryMBabcAlan D.IrvineMD, DScabcRachel M.McLoughlinPhDd
2019-1-17 review
创建过敏性疾病的科研、科普知识交流平台,为过敏患者提供专业诊断、治疗、预防的共享平台。

MK手机投注 | 安博·体育(中国)有限公司-官网 | 乐动官方网站 | 星空手机版 | 星空手机版 | mk体育(MKsports集团)股份公司 | 安博手机网页版登录入口 | 华体平台 | 千亿体育官网在线登录入口中国有限公司 |
 华亿体育(中国)游戏平台
华亿体育(中国)游戏平台

